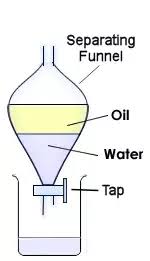
Separating funnel is a apparatus used in chemistry lab for separating two immiscible liquids.
Principle: The liquids to be separated differ in their densities. The more denser or heavier liquid makes the lower layer while the less denser liquid makes the upper layer.
Process: The two liquids are poured in the separating funnel and shaken sideways. Then, it is kept still for some time. The two liquids will start forming two layers. When the layers are fully separated, knob of the separating funnel is opened (a beaker/collector should already be placed ). The lower layer is collected in the collector. As soon as the first layer is poured completely in the collector, knob should be closed. Now, the denser layer has been collected into the collector and the lighter layer remains in the separating funnel.
Experimental procedure with an example:
Let us suppose that we need to separate oil and water.
Let us suppose that we need to separate oil and water.
- Pour the mixture of oil and water in the separating funnel, close it with the lid, and shake it well. (Not too vigorously)
- Keep it still for some time, say 5 min. ( You can place it on a tripod or burette stand.)
- When the 2 layers get separated, place a beaker/conical flask underneath it, and open the knob of the separating funnel.
- Collect the lower layer (water layer) in the beaker/conical flask.
- When the lower layer is completely poured, close the knob.
- Now, the water is collected in beaker and oil remained in the separating funnel.
Other examples: water + organic solvents (dichloromethane, chloroform, ethyl acetate) etc.
Advantages:
- Easy to handle
- Saves time
Disadvantages:
- The process is not 100% efficient. A small amount of lower layer kept in the separating funnel.
- It can separate only immiscible liquids which differ in their densities.