The matter is anything that has mass and occupies space. Matter exists in different phases, also known as states of matter.
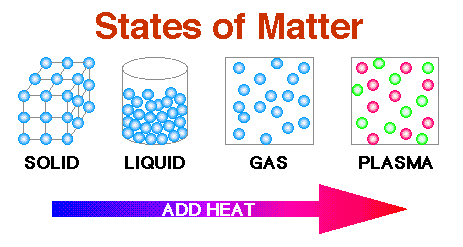
Theoretically, more than 27 states of matter are known, most of which are condensates, i.e., exist at a very low temperature or under drastic conditions. But only 5 states are well-established. These are solid, liquid, gas, plasma and bose-einstein. These are classified according to the force of attraction between the particles.
1. Solid: In this, particles are packed very tightly. As a result, they have
a. strong force of attraction.
b. fixed shape, size, and volume.
c. least compressible.
d. negligible or no diffusion.
a. strong force of attraction.
b. fixed shape, size, and volume.
c. least compressible.
d. negligible or no diffusion.
2. Liquids: In liquids, particles are packed comparatively loosely. As a result, they do not have strong force of attraction between the particles. They can
a. take the shape of the container in which it is poured.
b. be compressed when pressure is applied.
c. show the process of osmosis.
a. take the shape of the container in which it is poured.
b. be compressed when pressure is applied.
c. show the process of osmosis.
3. Gas: The particles in gases are very far apart since they have a little force of attraction between the particles. As a result, they are
a. highly compressible.
b. occupy the whole container.
c. highly diffusible.
a. highly compressible.
b. occupy the whole container.
c. highly diffusible.
The liquids and gases are known as fluids (to flow).
4. Plasma: Though this state of matter is not so common on our planet, it is the most common state of matter that is found in our universe. For example, stars, sun etc. Today, various inert gases are ionized using electricity so as to make glowing sign boards.
5. Bose-einstein condensate: In this, the atoms are supercooled to the temperature at which all the molecular motion ceases. At this stage, the atoms start to join together and form a cluster of atoms known as a super atom. They are also known as superfluids as they can flow without friction.
This state was named after two renown scientist, i.e., Albert Einstein and Satyendra Nath Bose.
This state was named after two renown scientist, i.e., Albert Einstein and Satyendra Nath Bose.
"Vapour is not a state of matter."
Vapour is an equilibrium state between the gaseous state and the liquid or solid state, which can come back to its original state when pressure is applied on it, keeping the temperature constant or at standard condition of pressure and temperature.
*Description of every state will be posted later in this blog.

No comments:
Post a Comment